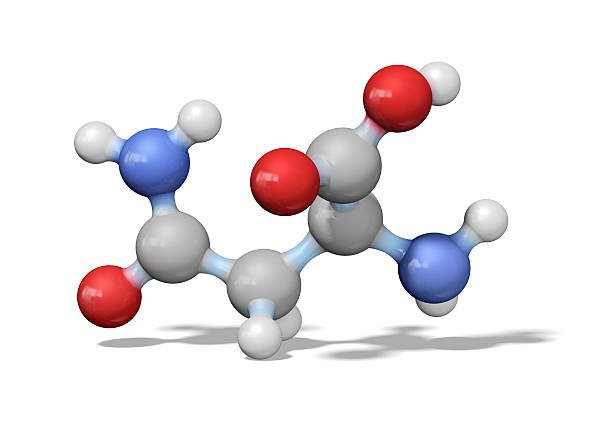
What characteristic would the r groups of amino acids have if they are located? Amino acids are the building blocks of proteins, playing a crucial role in a wide range of biological processes. The primary structure of a protein is determined by the linear sequence of amino acids linked together by peptide bonds. Each amino acid consists of a central carbon (C) atom, an amino group (NH2), a carboxyl group (COOH), and a unique side chain, also known as the R-group. The R-groups are responsible for the specific characteristics of amino acids, including their solubility, reactivity, and structural properties. In this article, we will discuss the characteristics of R-groups in amino acids depending on their location within a protein.
What characteristic would the r groups of amino acids have if they are located?
What characteristic would the r groups of amino acids have if they are located:
- Surface-exposed R-groups
Amino acids with R-groups located on the surface of a protein are typically involved in interactions with other molecules, including substrates, cofactors, and other proteins. The properties of these R-groups can determine the protein’s function, stability, and ability to form complexes with other biomolecules. Some common characteristics of surface-exposed R-groups include:
- Hydrophilic nature: Surface-exposed R-groups are often polar or charged, allowing them to interact with the aqueous cellular environment. Examples of such amino acids include serine, threonine, aspartic acid, and glutamic acid.
- Reactivity: Reactive R-groups on the protein surface can participate in catalysis or other chemical reactions. For example, cysteine can form disulfide bonds, while histidine can act as a catalytic residue in enzyme active sites.
- Flexibility: Surface-exposed R-groups can be flexible, allowing them to adopt different conformations to facilitate interactions with other molecules. Examples include the side chains of lysine and arginine, which are often involved in electrostatic interactions.
- Interior R-groups
Amino acids with R-groups located in the interior of a protein contribute to the protein’s overall structure and stability. The characteristics of these R-groups include:
- Hydrophobic nature: Interior R-groups are typically nonpolar and hydrophobic, promoting the formation of a hydrophobic core that stabilizes the protein’s three-dimensional structure. Common hydrophobic amino acids include alanine, valine, leucine, isoleucine, and phenylalanine.
- Rigid structure: Some interior R-groups have rigid structures that help maintain the protein’s overall conformation. Examples include proline, which induces kinks in the protein backbone, and tryptophan, which has a large, rigid indole ring.
- Aromaticity: Aromatic R-groups, such as those of phenylalanine, tyrosine, and tryptophan, can participate in π-π stacking interactions, contributing to the stability of the protein’s structure.
- R-groups at the N- and C-termini
The N-terminus and C-terminus of a protein are the amino and carboxyl ends of the polypeptide chain, respectively. The R-groups at these positions can have unique properties that influence protein folding, stability, and function. Some examples include:
- Terminal charges: The N-terminus typically carries a positive charge, while the C-terminus carries a negative charge. These charges can interact with other charged groups within the protein or with other molecules in the cellular environment.
- Post-translational modifications: R-groups at the N- and C-termini can undergo post-translational modifications, such as acetylation, methylation, or glycosylation, which can modulate protein function, stability, or localization.
Conclusion
The characteristics of R-groups in amino acids are influenced by their location within a protein, playing a critical role in determining protein function, stability, and interactions with other molecules. Surface-exposed R-groups often exhibit hydrophilic properties, reactivity, and flexibility, facilitating interactions with the aqueous cellular environment and other biomolecules. In contrast, interior R-groups are generally hydrophobic, rigid, and sometimes aromatic, contributing to the formation of a hydrophobic core and overall structural stability.
R-groups at the N- and C-termini of a protein can have unique properties, including terminal charges and the potential for post-translational modifications. These characteristics can modulate protein function, stability, or localization within the cell. Understanding the properties of R-groups and their influence on protein structure and function is essential for advancing our knowledge of molecular biology, biochemistry, and protein engineering. This knowledge can be applied to the design of novel proteins, therapeutics, and biotechnological applications, with potential benefits for human health and industry.