Chemical Equilibrium Notes Grade 12, with Questions and Answers on pdf notes format (Physical Science Grade 12): Chemical equilibrium is the state of a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction. While a reaction is in equilibrium the concentration of the reactants and products are constant.
Chemical Equilibrium Notes in Grade 12 Covers the following Topics
- Relate the concept of equilibrium to physical and chemical systems. Include: conditions necessary to achieve equilibrium
- Write equilibrium law expressions from balanced chemical equations for heterogeneous and homogeneous systems. Include: mass action expression
- Use the value of the equilibrium constant (Keq) to explain how far a system at equilibrium has gone towards completion.
- Solve problems involving equilibrium constants.
- Perform a laboratory activity to determine the equilibrium constant of an equilibrium system.
- Use Le Châtelier’s principle to predict and explain shifts in equilibrium. Include: temperature changes, pressure/volume changes, changes in reactant/product concentration, the addition of a catalyst, the addition of an inert gas, and the effects of various stresses on the equilibrium constant
- Perform a laboratory activity to demonstrate Le Châtelier’s principle.
- Interpret concentration versus time graphs. Include: temperature changes, concentration changes, and the addition of a catalyst
- Describe practical applications of Le Châtelier’s principle. Examples: Haber process, hemoglobin production at high altitude, carbonated beverages, eyes adjusting to light, blood pH, recharging of batteries, turbocharged/supercharged engines, ester synthesis, weather indicators, arrangement of produce, carbonated beverages in a hen’s diet . . .
- Write solubility product (Ksp) expressions from balanced chemical equations for salts with low solubility.
- Solve problems involving Ksp. Include: common ion problems
- Describe examples of the practical applications of salts with low solubility. Examples: kidney stones, limestone caverns, osteoporosis, tooth decay . . .
- Perform a laboratory activity to determine the Ksp of a salt with low solubility.
Downloadable Notes on pdf
Watch: Chemistry | Chemical equilibrium | Le Chartelie’s Principle
List of Chemical Equilibrium Grade 12 Questions and Answers Notes on pdf downloadable format
Chemical Equilibrium Test for Grade 12
The test (question bank) below gives the Grade 12 learners extra practice problems for revisions. The test covers the following: Equilibrium Conceptual, Writing the Equilibrium Constant, Manipulations of K: Reversing or Multiplying, Solving for K given all equilibrium concentrations, solving for an equilibrium concentration given K and other equilibrium concentrations, Using Ice, and more.
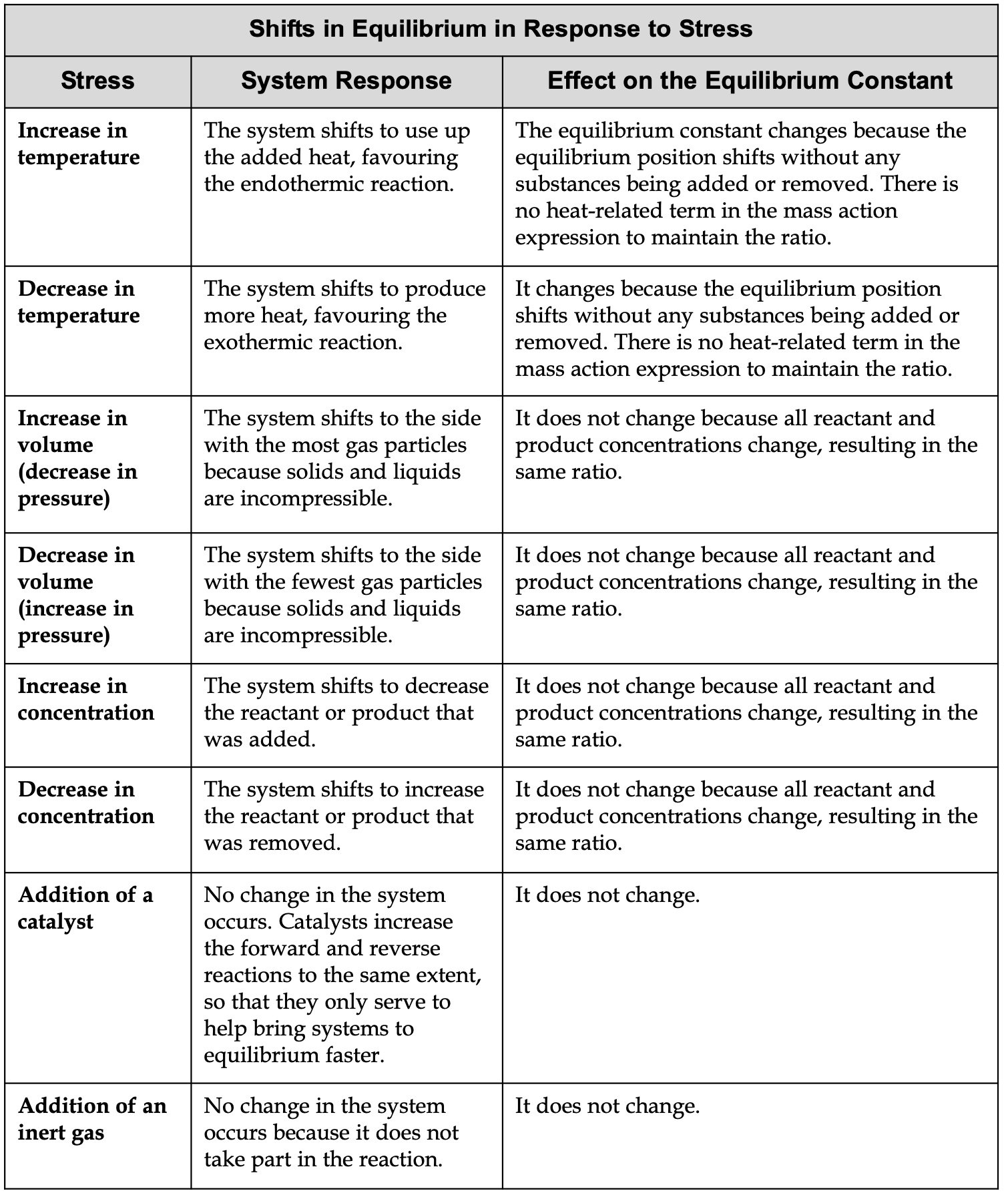
Shifts in Equilibrium in Response to stress Table
Below is the table which shows how does a system at equilibrium adjust to the changes in temperature, Volume, Concentration, Catalyst, and Inert Gas:

View all #Physical-Sciences-Grade 12 Study Resources
We have compiled great resources for Physical Sciences Grade 12 students in one place. Find all Question Papers, Notes, Previous Tests, Annual Teaching Plans, and CAPS Documents.