This page contains Organic Chemistry Grade 12 Notes on pdf formats which you can download. About a third of the Physical Sciences Grade 12 Examination Paper 2 comprises Organic Chemistry questions
Two most important parts about Organic Chemistry Grade 12 are:
- naming the organic molecules and learn the rules of naming molecules;
- understanding the reaction of organic molecules. Pay attention to the names of the reactions, as they provide some explanation about the reactions, e.g. e.g.: hydration means a water (hydro) molecule is added to the molecule in question; dehydration means the water molecule is removed.
Organic Chemistry is an important topic in the Grade 12 Physical Sciences curriculum in South Africa. It is the study of carbon-containing compounds, their structure, properties, and reactions. Here are the main areas covered in Organic Chemistry in the CAPS curriculum:
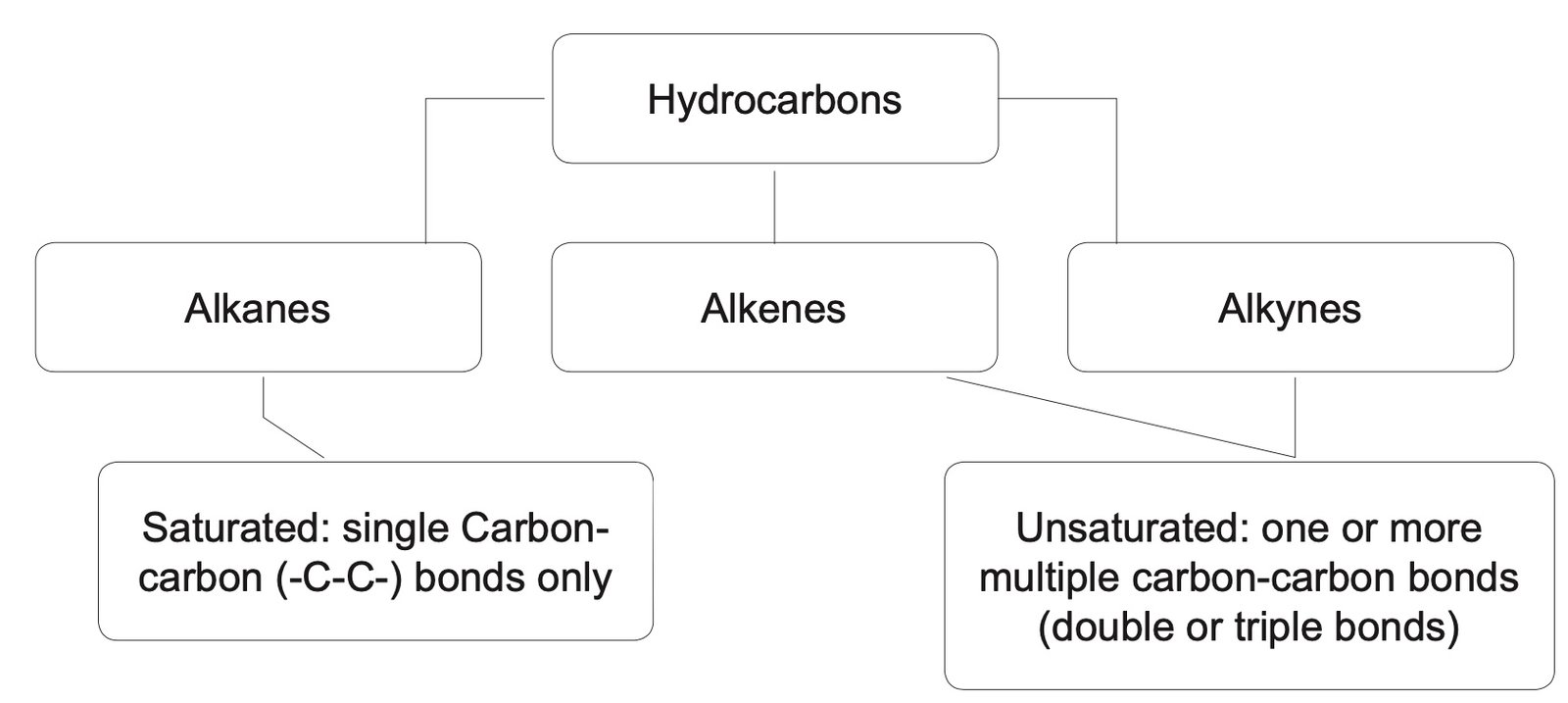
- Functional groups: Students learn about the different functional groups of organic compounds, including alkanes, alkenes, alkynes, alcohols, carboxylic acids, esters, and amines.
- Organic reactions: Students learn about the chemical reactions that occur between different organic compounds, including substitution, addition, elimination, and condensation reactions.
- Nomenclature: Students learn how to name organic compounds according to the IUPAC (International Union of Pure and Applied Chemistry) system.
- Isomerism: Students learn about the different types of isomerism, including structural isomerism, stereochemistry, and optical isomerism.
- Polymerization: Students learn about the formation of polymers from monomers, including addition polymerization and condensation polymerization.
- Organic analysis: Students learn about the techniques used to identify and analyze organic compounds, including infrared spectroscopy and mass spectrometry.
Overall, the Organic Chemistry topic in the Grade 12 Physical Sciences curriculum in South Africa aims to provide students with a fundamental understanding of the chemistry of carbon-containing compounds and their relevance to everyday life.
Video Lesson: Grade 12 Chemistry: Organic Chemistry
Atomic Theory: its Place in Organic Chemistry Grade 12
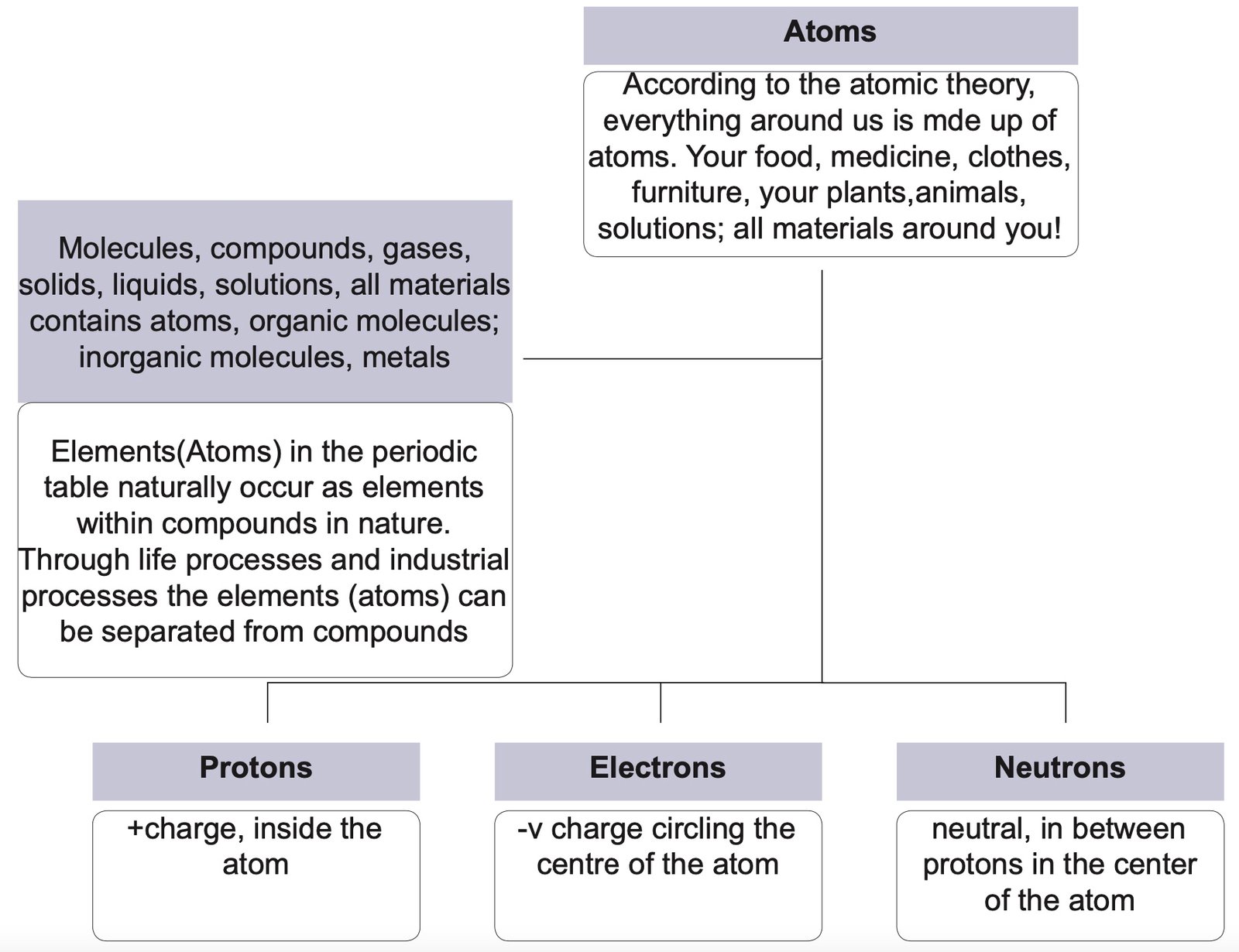
Organic compounds comprise the largest group of compounds on earth. Many things around you have carbon as the most abundant atom in them, including food, clothes, paper, coal, fuel, diamonds … the list is endless.

View all #Physical-Sciences-Grade 12 Study Resources
We have compiled great resources for Physical Sciences Grade 12 students in one place. Find all Question Papers, Notes, Previous Tests, Annual Teaching Plans, and CAPS Documents.
The carbon atom has wonderful properties that make it bond easily with other carbon atoms and with other atoms to form short chains and long chains. From methane, a dominant constituent of marsh gas to polymers …
A long time ago, clothes were made from cotton only, which is made up of carbon atoms. The second industrial revolution brought about the process of synthesis, when people realised
that they can make new products from existing materials and turn the material into something completely new through a range of reactions that usually mimic something that already exists naturally. Polymers are such an example. To a large extent, they have replaced cotton through reactions that produce synthetic materials such as polyester, polyamides, etc. These play an important role in the clothing industry, and in many other industrial and home products.
In South Africa, SASOL produced petrol out of coal. That is the beauty of synthesis: petrol from coal and not from oil!
Classification of hydrocarbons