On this page we discuss how the bulb is able to produce light.
The Science Behind the Light Bulb: How it Produces Light
The simple light bulb, an object that’s ubiquitous in our modern world, has an intricate science behind it. Whether it’s the warm glow of the incandescent bulb or the cool brightness of an LED, the processes by which bulbs produce light are both fascinating and varied. Let’s explore the heart of these light-producing mechanisms.
How the Bulb is Able to Produce Light
Light bulbs produce light through various methods depending on their type. In incandescent bulbs, a tungsten filament heats up when an electric current passes through it, causing it to glow and emit visible light due to the principle of ‘black body radiation.’ Compact Fluorescent Lamps (CFLs) contain a mix of argon gas and mercury vapor. When electrified, this mixture produces ultraviolet (UV) light, which then strikes a phosphorescent coating inside the bulb, causing it to glow with visible light through a process called ‘fluorescence.’ On the other hand, Light Emitting Diodes (LEDs) are composed of semiconductor materials. When an electric current passes through an LED, electrons move and recombine with electron holes, releasing energy in the form of photons, which we perceive as light. The specific color or wavelength of the light from an LED is determined by the semiconductor’s energy gap.
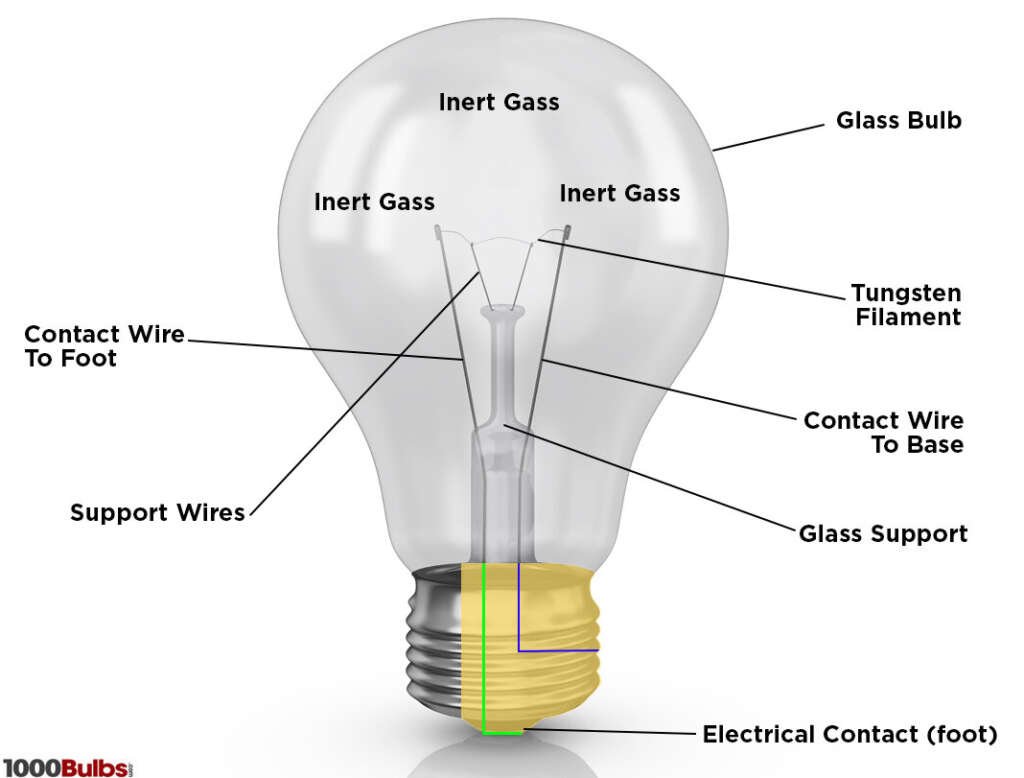
1. Incandescent Bulbs
The Mechanism: The primary component inside an incandescent bulb is a thin tungsten wire, known as a filament. When electrical current passes through this filament, the resistance of the wire causes it to heat up. As it reaches higher temperatures, it emits visible light.
The Science: This phenomenon is based on the principle of ‘black body radiation.’ As objects, particularly metals, become hot, they emit radiation. Initially, at lower temperatures, the radiation is in the infrared spectrum, which is invisible to the human eye. However, as the temperature increases, the radiation shifts into the visible spectrum, and we see it as light.
2. Compact Fluorescent Lamps (CFLs)
The Mechanism: CFLs contain a gas, usually argon mixed with a small amount of mercury vapor. When an electric current passes through this gas mixture, it energizes the mercury vapor and produces ultraviolet (UV) light. The UV light then strikes a phosphorescent coating on the inside of the bulb, causing it to glow and produce visible light.
The Science: The process of converting UV light into visible light through the interaction with a phosphorescent coating is called ‘fluorescence.’ This phenomenon is the reason behind the name ‘fluorescent lamp.’
3. Light Emitting Diodes (LEDs)
The Mechanism: LEDs are made of semiconductor materials. When an electric current passes through an LED, it causes electrons to move and recombine with electron holes. As this happens, energy is released in the form of photons, producing light.
The Science: The color (or wavelength) of the light produced by an LED depends on the energy gap of the semiconductor. Thus, different materials can produce different colors of light. Over the years, advancements in semiconductor technology have allowed LEDs to cover a broad spectrum of colors, including the white light commonly used in household lighting.
Conclusion
While the end result of a bulb is to produce light, the means by which different bulbs achieve this varies greatly. From the heated filaments of incandescent bulbs to the semiconductor magic of LEDs, each technology harnesses unique physical principles to illuminate our world. As technology advances and the need for energy-efficient solutions grows, we may witness further innovations in the realm of light production. Regardless of the method, the science behind it remains a testament to human ingenuity and our quest to conquer the darkness.

